Topics
Our group aims to understand the mechanisms leading to loss of bone tissue integrity and to abnormal calcification of soft tissues in humans and mice. Accordingly, our research projects focus on the cellular and molecular mechanisms involved in skeletal formation and mineralization, the interaction between bone and other skeletal tissues, and the role of phosphate sensing. The ultimate goal is to identify novel strategies for fracture prevention, fracture healing and bone regeneration, and be able to counteract the deleterious effect of phosphate in pathophysiological conditions such as chronic kidney disease.
We recently obtained an ANR funding entitled « Phosphate and vascular calcifications in chronic kidney disease » (PARKA project) whom overall objective is to uncover the mechanism of action of Pi resulting in the development of vascular calcifications in the context of CKD, and identify putative therapeutic targets capable of counteracting its deleterious effects.
Scientific Approach
To explore these topics, we are deciphering the roles and mechanisms of action of PiT1 and PiT2 proteins in the skeleton and related tissues using genetic approaches in mice. PiT1 and PiT2 were originally described as phosphate transporters, but we have shown that they are in fact multifunctional proteins. Exploring the physiology and pathophysiology of the skeleton through the study of PiT’s mechanisms of action brings us to study both phosphate-related and unrelated phenomenons.
Main findings
Skeletal and teeth mineralization
|
 |
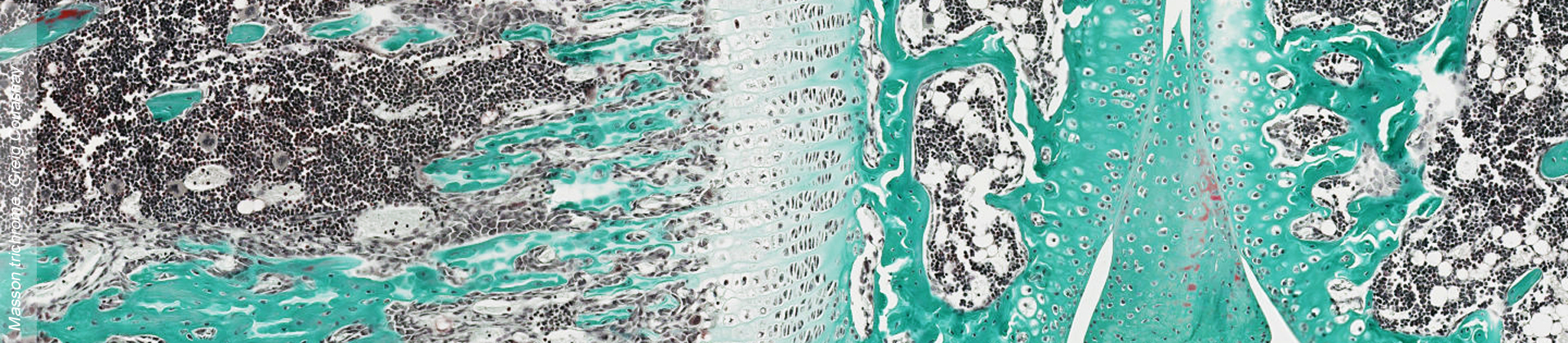
PiT1 and PiT2 are expressed in a wide range of tissues and are the only known phosphate transporters expressed in the skeleton. For this reason, it has long been assumed that PiTs might play an important role in skeletal mineralization, but no data in the literature was supporting this hypothesis.
Using genetic approaches in mouse, we have shown for the first time that PiT2, but surprisingly not PiT1, is an important factor in bone mineralization, strength and quality in mice.
Beck-Cormier S et al., Journal of Bone and Mineral Research, 2019, https://doi.org/10.1002/jbmr.3691
Merametdjian L et al., Journal of Dental Research, 2018, https://doi.org/10.1177/0022034517729811
Yadav MC et al., Journal of Bone and Mineral Research 2016, https://doi.org/10.1159/000369742
Bourgine A et al., PLoS ONE, 2013, https://doi.org/10.1371/journal.pone.0065979
|
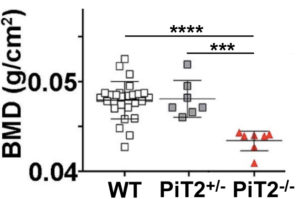 |
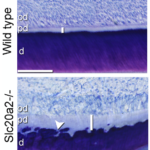
Bone quality
|
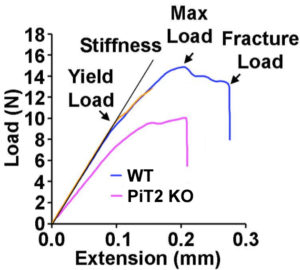 |
We specifically identified PiT2 as a marker of bone quality and strength. Bones from mice lacking PiT2 have reduced strength and stiffness. Interestingly, the effect of PiT2 deletion does not appear to be bone-autonomous but is probably due to an interaction with other skeletal tissues.
Beck-Cormier S et al., Journal of Bone and Mineral Research, 2019, https://doi.org/10.1002/jbmr.3691
|
Chondrocyte survival
|
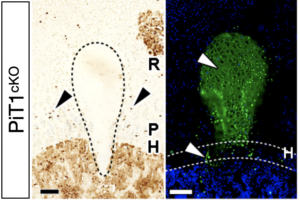 |
Although PiT1 is not obligatory for skeletal mineralization as was long thought, we showed that its crucial role in the skeleton was to promote chondrocyte survival during long bone growth. This role is not related to its transport activity but rather to its ability to bind and modulate protein disulfide isomerase (PDI) that is essential for protein folding within the endoplasmic reticulum (ER). Therefore, the absence of PiT1 causes an intense ER stress due to the accumulation of misfolded proteins, and also leads to a specific retention of the survival factor VEGF-A in the ER, causing increased chondrocyte death.
Couasnay G et al., Journal of Bone and Mineral Research, 2019, https://doi.org/10.1002/jbmr.3609 |
Phosphate sensing
|
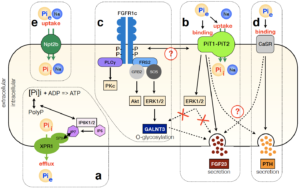 |
PiT1 and PiT2 have unique transporting characteristics, which are actually not fully compatible with the requirements of large quantities of phosphate ions for mineralization purposes (low Vmax, low Km). In addition, PiT1 and PiT2 share similar structural protein arrangements. Based on these informations, we were the first team showing that PiT1 and PiT2 proteins can heterodimerize at the plasma membrane. We showed that this heterodimerization in dependent on extracellular phosphate variations and that they act as a Pi sensor complex.
Beck L & Beck-Cormier S. Journal of Molecular Endocrinology 2020, https://doi.org/10.1530/JME-20-0121
Bon N et al, Current Molecular Biology Reports, 2019, https://doi.org/10.1007/s40610-019-0109-2
Bon N et al., Molecular Metabolism, 2018, https://doi.org/10.1016/j.molmet.2018.02.007
Bon N et al., Journal of Biological Chemistry, 2018, https://doi.org/10.1074/jbc.M117.807339 |
PiT1 and PiT2 are multifunctional proteins
|
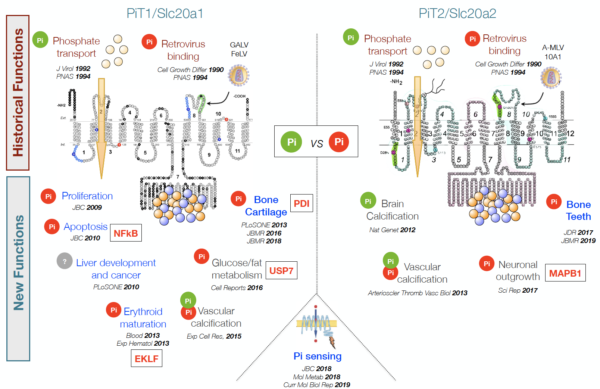 |
Originally described as phosphate transporters, we were the first group (Beck L et al., JBC 2009) to describe these proteins as having additional phosphate transport independent functions (symbolized by a Pi red dot in the figure on the left).
Our group, and others now, has shown that these functions are involved in a wide variety of physiological situations: cancer cell proliferation, programmed cell death, erythrocyte maturation, liver development, liver cancer, vessel formation, brain calcification, neurite growth, etc. A very interesting aspect of our research is to understand the molecular basis of this diversity.
Beck-Cormier S, Beck L. Journal of Bone and Mineral Research 2020, 35(4): 825-6, https://doi.org/10.1016/j.celrep.2016.08.012
|
Ongoing work
|

NanoCT of a mouse femur showing the bone microarchitecture (white) and the bone marrow adiposity (red).
Credits: Giulia Frangi
|
Our current work further explores the role of PiT1 and PiT2 in particular physiological and pathophysiological situations.
We focus on deciphering their roles and their mechanism of action in the interaction between bone and other skeletal tissues (vessels and bone marrow adiposity), as well as the sensing of phosphate in vascular calcification and other cardiovascular diseases.
|
NanoCT reconstruction of a mouse femur showing the bone microarchitecture (white) and the bone vessels (red).
Credits: Giulia Frangi
|